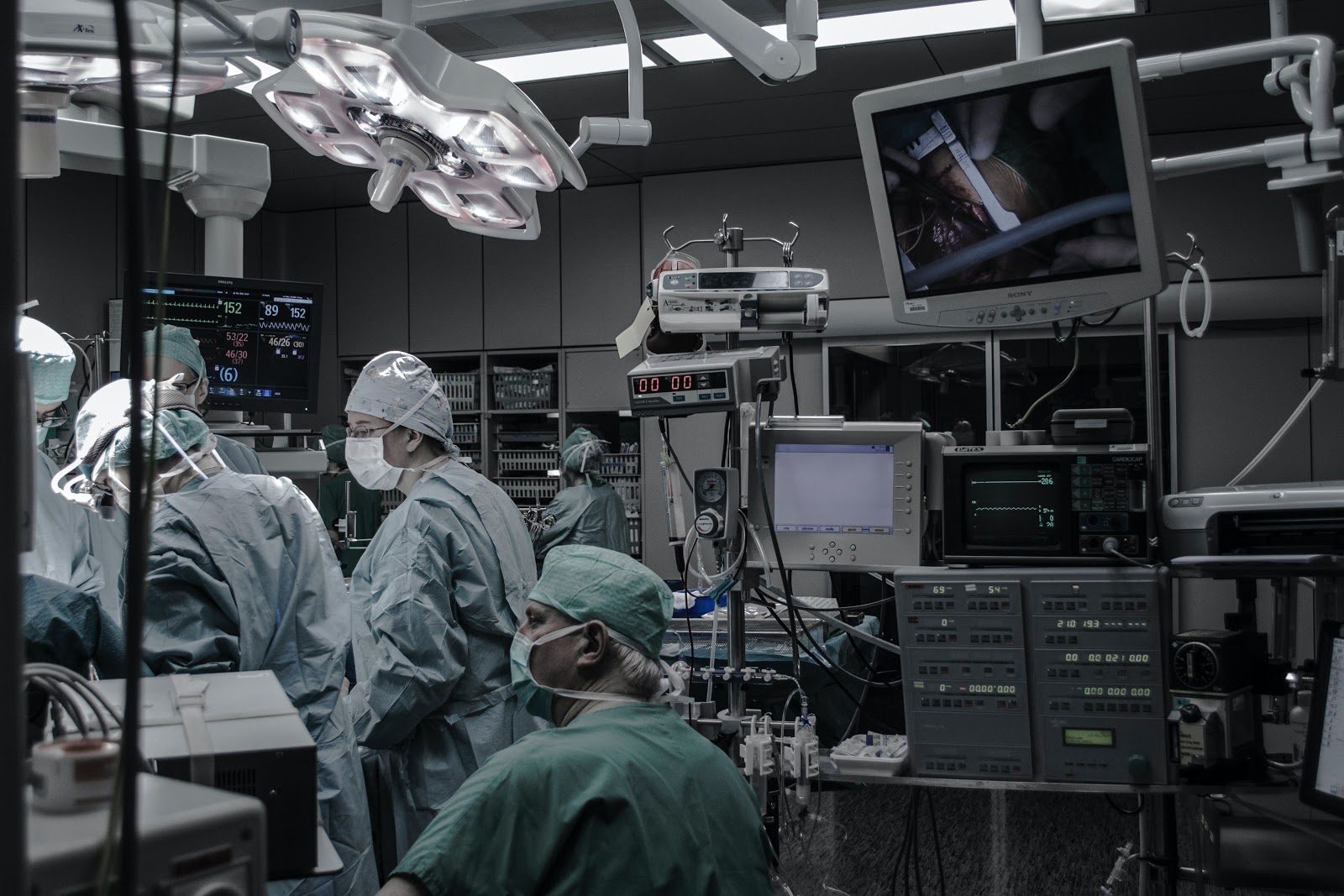
According to a recent report by The Lancet Commission, AI-powered medical devices are poorly regulated in the European Union and the United States.
Recently, scientists from University of Zurich have published a report on the approval process of medical devices in the EU and the US. The review exposed glaring loopholes in the process.
Here, we try to analyse the failures of the EU and the US governments in regulating AI-powered medical devices. We will also delve into the regulation of such devices in India and what needs to be done to improve it.
What Is Happening In The US & The EU?
Majority of the AI-powered medical devices in the EU and the US are used by radiologists to interpret CT and MRI scans. The US and the EU have approved a substantial number of medical devices in the last five years as the clinical adoptions increase.
However, according to the study, out of 462 approvals, only five devices in the EU and the US sufficiently demonstrated safety and effectiveness.
The US has a centralised approval system, where the Food & Drugs Association (FDA) solely maintains a repository of all the approved medical devices. However, it still does not have a specific pathway to approve AI-based medical devices.The most stringent ‘premarket approval’ process was used for only three of the 222 devices cleared in the US.
However, the US has mooted an action plan to regulate AI-powered medical devices based on the product’s lifecycle.
Meanwhile, in Europe, the approvals are not centralised. The EU-27, Norway, Iceland, Liechtenstein, Switzerland and Turkey are allowed to approve medical devices individually. Approvals are based on the device’s classification, from Class I to III. Class III has the most stringent rules for approval.
Interestingly, only two devices out of the 240 were approved using Class III. Moreover, the approval of Class II and III can be done by Private Notified Bodies who can confer Conformité Européenne (CE) mark to the devices. Less than two in three devices were approved using this mark.
India’s Status
India’s medical devices sector saw an inflow of FDI worth $1.8 billion from 2000 to 2019, with the market size expected to hit $50 billion by 2025. Forget AI-powered medical devices space, India lacks the infrastructure and legal frameworks for AI in general.
In India, medical devices and drugs used to be under the same category until recently. A clear distinction was made only in 2017 under the new Medical Device Rules. However, it did not take into account softwares used for medical purposes under its purview. In 2020, an amendment was introduced. The change in law also mandated ISO certifications for importers or manufacturers of the device.
While compliance with international norms does ensure some quality, the proposed framework still does not have specific provisions to deal with the dynamic demands of emerging technologies, the research showed. At the same time, adopting Western standards and mechanisms for AI-based medical devices might limit AI innovations in healthcare, given the country’s unique ecosystem.
India does not have a framework to classify various medical devices based on a clinical understanding or AI modalities. Different levels of approvals, depending on a ‘risk-assessment framework’, can drive innovation. Based on the framework, the deployment of low-risk medical devices can be prioritised, and stringent regulation around high-risk medical devices can be ensured.
It is also essential to take stock of the AI-enabled medical device’s interaction with humans, especially for high-risk machines. The human element should be accounted for in the approval process to do a more holistic assessment of the medical devices.
Lastly, under the Medical Devices Rules, the US’ FDA or EU’s CE certified medical devices can be marketed in India without undergoing clinical trials. In the light of The Lancet Commission’s study, this is worrisome, and India needs to consider the various categories of FDA and CE approvals in detail. This is especially true in CE-approved devices since 12 CE approvals were found to be unsafe or ineffective by the FDA.
Wrapping Up
AI is making huge inroads into the healthcare industry. It presents immense potential, especially in a country like India, with a flawed healthcare system. However, as medical devices enter the market, it is also essential to ensure their effectiveness and safety.
Countries worldwide need to develop effective standardisation frameworks for AI-based medical devices with the right balance of innovation and safety.